Specialized Sounds in Cetacean Communication
In water, sound typically propagates farther than light. Thus, it is not surprising that acoustic communication, defined as transmission of information from a signaling animal to a receiving animal, plays a key role in the behavior and social interactions of marine animals, especially marine mammals. Marine mammals, like cetaceans that live in cohesive social groups, need to communicate in order to coordinate their activities and maintain social ties.

Communication signals of some dolphin species have been well-studied. Dolphins, such as these spinner dolphins, produce whistles as a form of communication. Scientists have been cataloging and categorizing whistles from various study populations for some time. Image credit: NOAA Fisheries.
Cetaceans produce a variety of tonal and pulsed calls for underwater communication. To understand the function of these sounds, scientists must identify which animal is producing a given sound, the responses of animals that hear it, and the context under which the signal is produced. The specific structure of the signal and propagation characteristics are also important.
Scientists, through both field observations and experiments with captive animals, have found that the types of social bonds between individuals in a group correlate with specific types of communication signals:
- Individual-specific vocalizations are used by species with strong, individual, social bonds such as bottlenose dolphins.
- Group-specific vocalizations are used by social species with stable groups such as killer whale pods, sperm whale clans, and short-finned pilot whale pods in Hawaii.
- Population-specific vocalizations are used by species that appear to have neither long-term social bonds nor stable groups. For example, humpback whale song, which is associated with mating, is similar within an ocean basin but differs between ocean basins.
In general, echolocation is not considered a form of communication, but is used for navigation and hunting. However, it may be possible that animals can glean information from other animals’ echolocation clicks[1]Xitco, M. J., & Roitblat, H. L. (1996). Object recognition through eavesdropping: Passive echolocation in bottlenose dolphins. Animal Learning & Behavior, 24(4), 355–365. https://doi.org/10.3758/BF03199007[2]Gregg, J. D., Dudzinski, K. M., & Smith, H. V. (2007). Do dolphins eavesdrop on the echolocation signals of conspecifics? International Journal of Comparative Psychology, 20(1). https://escholarship.org/uc/item/20s5h7h9. For example, sperm whale clicks, while primarily intended for echolocation, can be arranged to form a communication structure called a coda[3]Watkins, W. A., & Schevill, W. E. (1977). Sperm whale codas. The Journal of the Acoustical Society of America, 62(6), 1485. https://doi.org/10.1121/1.381678.
Cetaceans produce a variety of underwater sounds for communication including the tonal and pulsed calls mentioned above. Several of these specialized sounds are described below. In some cases, including signature whistles and contact calls, considerable evidence exists for a specific communication role for these sounds. There are many other categories of cetacean sounds, such as burst pulsed calls, click communication, and deep-water tonal sounds, for which there is some evidence of a communicative role, but a specific function has not been determined.
Whistles
Toothed cetaceans produce vocalizations over a broad range of frequencies. They also exhibit acoustic plasticity, adapting signal structure in response to natural or anthropogenic factors, the state of an animal, and social context.
One of the most well-studied odontocete vocalizations is the whistle, a frequency modulated, narrow-band signal. Whistles last from several tenths of a second to several seconds and typically range in fundamental frequency between 2 and 25 kHz. Some dolphin species, such as the Guiana dolphin, can produce very high frequency whistles (> 40 kHz) that may be intended for communication across short distances[4]Barbosa, M., Bittencourt, L., G. Andrade, L., L. Bisi, T., Lailson-Brito, J., & F. Azevedo, A. (2019). High-frequency social communication in Sotalia guianensis. The Journal of the Acoustical Society of America, 146(2), EL124–EL128. https://doi.org/10.1121/1.5120550.. Each species produces distinctive whistle features, which help scientists identify species[5]Oswald, J. N., Rankin, S., Barlow, J., & Lammers, M. O. (2007). A tool for real-time acoustic species identification of delphinid whistles. The Journal of the Acoustical Society of America, 122(1), 587–595. https://doi.org/10.1121/1.2743157..
Signature whistles are one type of individual-specific communication signal. Many dolphin species, such as bottlenose dolphins, common dolphins, Pacific white-sided dolphins, Pacific humpback dolphins, and spotted dolphins, produce signature whistles. They are thought to identify individuals in many situations, such as when different groups meet at sea or when mother/calf pairs are separated.
During the first year of life, a dolphin develops its signature whistle based on other whistles in its environment (“vocal learning”). Once developed, the dolphin’s signature whistle usually does not change significantly. This whistle stability is thought to foster long-term social bonds within dolphin groups. During field-based playback experiments, scientists found bottlenose dolphins to not only discriminate between different whistles of familiar individuals, but also show a significant likelihood of head-turning in response to the playback of whistles from a mother or independent offspring[6]Sayigh, L. S., Tyack, P. L., Wells, R. S., Solow, A. R., Scott, M. D., & Irvine, A. B. (1999). Individual recognition in wild bottlenose dolphins: A field test using playback experiments. Animal Behaviour, 57(1), 41–50. https://doi.org/10.1006/anbe.1998.0961..
Frequency cues (differences in the frequency contours of a signal between individuals) are critical elements of individual signature whistles; some dolphins can detect as little as a 0.2% change in a signal’s frequency[7]Tyack, P. L., & Clark, C. W. (2000). Communication and Acoustic Behavior of Dolphins and Whales. In W. W. L. Au, R. R. Fay, & A. N. Popper (Eds.), Hearing by Whales and Dolphins (Vol. 12, pp. 156–224). Springer New York. https://doi.org/10.1007/978-1-4612-1150-1_4.. Dolphins can also mimic the signature whistles of other individuals of their species. This vocal matching appears to occur between animals that share strong social bonds. Signature whistles are a good illustration of “vocal matching.” This is a behavior where a listening animal responds to a signal of a near-by animal by changing some of the features of its own response in order to imitate the signal from the other animal. The timing of this response is a crucial factor in vocal matching and may indicate the motivation of the respondent. For example, if there is a long pause after the initial signal, the subsequent response may not be recognized as a reply to that signal. Playback experiments with bottlenose dolphins showed an optimal time interval for vocal matching of signature whistles to be less than 1 second[8]King, S. L., Harley, H. E., & Janik, V. M. (2014). The role of signature whistle matching in bottlenose dolphins, Tursiops truncatus. Animal Behaviour, 96, 79–86. https://doi.org/10.1016/j.anbehav.2014.07.019..
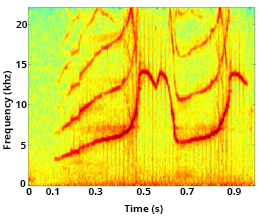
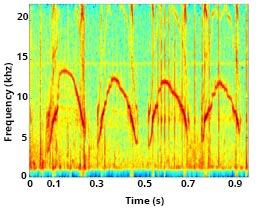
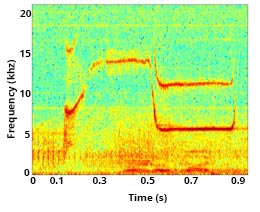
Contact Calls
Most cetaceans have evolved specific communication signals, including contact calls, to maintain connection and/or reunite with one another. A most basic and common bond in mammals is between a mother and her young. As a calf matures, the proportion of active behaviors (travel, foraging, and social activity) increases, resulting in physical separation between mother and calf. Contact calls are an important form of communication to signal the desired return of the calf to the mother.
Signature whistles (discussed above) can serve as a type of contact call and play an important role in facilitating mother-calf coordination. Mother bottlenose dolphins are more likely to use acoustic signals to recall their calf, rather than pursue their young and physically herd them to a location. In addition to their signature whistle, mother dolphins may also produce other secondary whistles, and occasionally clicks, to recall their calf. The mother’s call communicates information about her location and/or motivational state, and/or emphasizes the need for the calf to return[9]Kuczaj, S., Eskelinen, H., Jones, B., & Borger-Turner, J. (2015). Gotta Go, Mom’s Calling: Dolphin (Tursiops truncatus) Mothers Use Individually Distinctive Acoustic Signals To Call Their Calves. Animal Behavior and Cognition, 2(1), 88–95. https://doi.org/10.12966/abc.02.07.2015. [10]King, S. L., Guarino, E., Keaton, L., Erb, L., & Jaakkola, K. (2016). Maternal signature whistle use aids mother-calf reunions in a bottlenose dolphin, Tursiops truncatus. Behavioural Processes, 126, 64–70. https://doi.org/10.1016/j.beproc.2016.03.005..
Other cetaceans produce sounds that seem to function as contact calls. Broadband, pulsed contact calls have been described for beluga and narwhal whales[11]Shapiro, A. D. (2006). Preliminary evidence for signature vocalizations among free-ranging narwhals (Monodon monoceros). The Journal of the Acoustical Society of America, 120(3), 1695–1705. https://doi.org/10.1121/1.2226586.[12]Marcoux, M., Auger-Méthé, M., & Humphries, M. M. (2012). Variability and context specificity of narwhal (Monodon monoceros) whistles and pulsed calls. Marine Mammal Science, 28(4), 649–665. https://doi.org/10.1111/j.1748-7692.2011.00514.x.[13]Morisaka, T., Yoshida, Y., Akune, Y., Mishima, H., & Nishimoto, S. (2013). Exchange of “signature” calls in captive belugas (Delphinapterus leucas). Journal of Ethology, 31(2), 141–149. https://doi.org/10.1007/s10164-013-0358-0.[14]Vergara, V., & Mikus, M. (2019). Contact call diversity in natural beluga entrapments in an Arctic estuary: Preliminary evidence of vocal signatures in wild belugas. Marine Mammal Science, 35(2), 434–465. https://doi.org/10.1111/mms.12538. Exchanges of these calls between individuals resemble those of signature whistles; the sounds produced by captive beluga whales are individually distinct. In one study with wild belugas, the number of acoustically distinct contact calls was positively correlated with the number of individuals[15]Vergara, V., & Mikus, M. (2019). Contact call diversity in natural beluga entrapments in an Arctic estuary: Preliminary evidence of vocal signatures in wild belugas. Marine Mammal Science, 35(2), 434–465. https://doi.org/10.1111/mms.12538. These observations suggest that beluga whale contact calls may also function as an individual identification, like the signature whistle. However, it remains unknown whether individual identity is actually encoded in these calls.
Baleen whales also produce signals that may function as contact calls. For example, right whales produce the “upcall” which is a short, tonal, sweep that ascends in frequency. These vocalizations are produced by males and females of all age classes and are thought to be a primary contact call, including mothers with calves. Mother-calf pairs call at low rates on the calving grounds; however, call activity increases as pairs move to foraging areas and as calves increase in age and activity level. The highest calling rates occur during surface active play[16]Cusano, D. A., Conger, L. A., Van Parijs, S. M., & Parks, S. E. (2019). Implementing conservation measures for the North Atlantic right whale: Considering the behavioral ontogeny of mother‐calf pairs. Animal Conservation, 22(3), 228–237. https://doi.org/10.1111/acv.12457.. In general, rates of upcalls can be affected by behavioral state, with lower rates of upcalls produced during periods of foraging, traveling, or resting, and higher rates during surface activities.
Burst Pulsed Sounds
Although much marine mammal acoustic research has focused on whistles and other frequency-modulated signals, burst pulsed sounds are another common communication signal used by many species of toothed whales. These broadband, high frequency sounds with short inter-pulse intervals can be difficult to describe because their characteristics are highly variable. Burst pulsed sounds are often described as squawks, creaks, bleats, barks, and pops. They may be produced singly, in bouts, or in association with whistles. They also often occur in agonistic interactions and/or may signal a change in behavior. For example, “long burst pulsed sounds” (duration longer than 200 ms) produced by Sardinian bottlenose dolphins during depredation events may be used as avoidance vocalizations to prevent competition between group members[17]Díaz López, B., & Shirai, J. A. B. (2009). Mediterranean common bottlenose dolphin’s repertoire and communication use. In A. G. Pearce & L. M. Correa (Eds.), Dolphins: Anatomy, Behavior, and Threats. (pp. 129–148). Nova Science Publishers, Inc.. Researchers have found that burst pulsed sounds produced by white-beaked dolphins took place before a pod switched from slow swimming with frequent directional changes to rapid, straight-line swimming[18]Simard, P., Mann, D. A., & Gowans, S. (2008). Burst-Pulse Sounds Recorded from White-Beaked Dolphins (Lagenorhynchus albirostris). Aquatic Mammals, 34(4), 464–470. https://doi.org/10.1578/AM.34.4.2008.464..
Some odontocete species that do not produce whistles may use burst pulsed sounds for communication. For example, northern right whale dolphins produce stereotyped, repeated series of burst pulsed calls unique to the same subgroup of animals[19]Rankin, S., Oswald, J. N., Simonis, A. E., & Barlow, J. (2015). Vocalizations of the rough-toothed dolphin, Steno bredanensis , in the Pacific Ocean. Marine Mammal Science, 31(4), 1538–1548. https://doi.org/10.1111/mms.12226.. These calls may serve a similar communicative function as delphinid whistles or killer whale discrete calls.
Recording of burst pulsed sounds made by Hawaiian spinner dolphins (Stenella longirostris) from an environmental acoustic recording deployment off of Kahe Point, Oʻahu (21.35467, -158.1368) at a depth of 15.24 m on April 8, 2019 at 07:20 am. Sound recording by Megan M. McElligott and Marc O. Lammers with the University of Hawaiʻi at Mānoa and the Oceanwide Science Institute.
Click-Based Communication
Click-based communication has been well-studied for odontocete species, such as the sperm whale. Within a social unit, most sperm whales share common coda types. Codas are stereotyped sequences of broadband clicks which are differentiated by their rhythm or temporal pattern. Codas are thought to have a communicative function in maintaining social unit cohesion (e.g., following periods of dispersion during foraging). Sperm whale social units can be assigned to large-scale “vocal clans” based on the shared, stable usage of particular codas over time. Social units generally associate with other units of the same vocal clan[20]Rendell, L. E., & Whitehead, H. (2003). Vocal clans in sperm whales (Physeter macrocephalus). Proceedings of the Royal Society B: Biological Sciences, 270(1512), 225–231. https://doi.org/10.1098/rspb.2002.2239.. Codas may also have distinct features that contribute to individual identification[21]Oliveira, C., Wahlberg, M., Silva, M. A., Johnson, M., Antunes, R., Wisniewska, D. M., Fais, A., Gonçalves, J., & Madsen, P. T. (2016). Sperm whale codas may encode individuality as well as clan identity. The Journal of the Acoustical Society of America, 139(5), 2860–2869. https://doi.org/10.1121/1.4949478[22]Gero, S., Whitehead, H., & Rendell, L. (2016). Individual, unit and vocal clan level identity cues in sperm whale codas. Royal Society Open Science, 3(1), 150372. https://doi.org/10.1098/rsos.150372..
Porpoises also produce click-based communication signals. These toothed cetaceans produce narrow-band high frequency (NBHF) clicks to mediate social interactions such as mate choice and mother-calf contact. NBHF clicks provide a means for communication that is above the hearing range of potential predators, such as killer whales (“acoustic crypsis”). Acoustic recording tags placed on six wild harbor porpoises frequently detected NBHF clicks, in dense bouts, with repetition rates of up to 27 calls per minute. These calls were separated by silent periods. Although high call rates were noted in all tagged animals, a higher proportion of NBHF clicks were produced by mothers accompanied by a dependent calf[23]Sørensen, P. M., Wisniewska, D. M., Jensen, F. H., Johnson, M., Teilmann, J., & Madsen, P. T. (2018). Click communication in wild harbour porpoises (Phocoena phocoena). Scientific Reports, 8(1), 9702. https://doi.org/10.1038/s41598-018-28022-8.. Mother-calf porpoise pairs were also found to produce different click patterns based on behavior; porpoises may thus encode communicative information in call features such as click repetition rate (CRR)[24]Clausen, K. T., Wahlberg, M., Beedholm, K., Deruiter, S., & Madsen, P. T. (2011). Click communication in harbour porpoises Phocoena phocoena. Bioacoustics, 20(1), 1–28. https://doi.org/10.1080/09524622.2011.9753630..
The high frequency levels and directional nature of NBHF clicks results in a small space for conspecifics to detect and/or respond to emitted signals. High call rates coupled with short periods of silence between calls may indicate that porpoises compensate for this through repeatedly calling out in different directions to advertise their position and/or to establish contact[25]Sørensen, P. M., Wisniewska, D. M., Jensen, F. H., Johnson, M., Teilmann, J., & Madsen, P. T. (2018). Click communication in wild harbour porpoises (Phocoena phocoena). Scientific Reports, 8(1), 9702. https://doi.org/10.1038/s41598-018-28022-8..
Deep Water Communication
Deep depths create particular challenges for sound production. Increasing hydrostatic pressure with depth limits the duration and amplitude of tonal calls. However, the amplitude of echolocation clicks has not been found to be affected by depth[26]Jensen, F. H., Perez, J. M., Johnson, M., Soto, N. A., & Madsen, P. T. (2011). Calling under pressure: Short-finned pilot whales make social calls during deep foraging dives. Proceedings of the Royal Society B: Biological Sciences, 278(1721), 3017–3025. https://doi.org/10.1098/rspb.2010.2604..
Short-finned pilot whales have been found to produce tonal sounds during deep foraging dives. Their mean call amplitude and duration decreased with depth, even though increased distance to conspecifics at the surface would seem to require louder and longer calls[27]Jensen, F. H., Perez, J. M., Johnson, M., Soto, N. A., & Madsen, P. T. (2011). Calling under pressure: Short-finned pilot whales make social calls during deep foraging dives. Proceedings of the Royal Society B: Biological Sciences, 278(1721), 3017–3025. https://doi.org/10.1098/rspb.2010.2604.. Calls at depth tended to be simple, short downsweeps. Although these calls have been detected throughout all parts of foraging dives, short-finned pilot whales produced these sounds more often while ascending to the surface. The primary social groups of short-finned pilot whales often cluster together in larger aggregations in one area, and these deep calls may thus be used as group-specific cues to maintain or re-establish contact with other pilot whales at the surface.

Dive profile of a short-finned pilot whale tagged with a multisensory acoustic tag comprising a period at the surface followed by two deep foraging dives. Two tonal calls from the last ascent (see letters in dive profile, A is a shallow call, B a deepwater call at the bottom of the dive around 600m) are shown as inset waveforms and spectrograms. Note the lower amplitude and shorter duration of the deep call (B). In the dive profile graphic, grey lines represent regular echolocation clicking; red dots, tonal calls; and green dots, pulsed calls. Adapted with permission from Jensen et al. 2011. Calling under pressure: short-finned pilot whales make social calls during deep foraging dives. Proceedings of the Royal Society B. 278: 3017–3025.
Blainville’s beaked whales also produce tonal calls at depth. However, they live in smaller groups than short-finned pilot whales and are silent 80% of the time during foraging dives. Blainville’s beaked whales only produce sounds at depths greater than 170m, and are silent during ascents[28]Aguilar de Soto, N., Madsen, P. T., Tyack, P., Arranz, P., Marrero, J., Fais, A., Revelli, E., & Johnson, M. (2012). No shallow talk: Cryptic strategy in the vocal communication of Blainville’s beaked whales. Marine Mammal Science, 28(2), E75–E92. https://doi.org/10.1111/j.1748-7692.2011.00495.x.. Tonal sounds are produced most often around the beginning of the vocal phase of deep dives, when the whales separate to forage at depth. This behavior is thought to limit the ability of shallow‐diving predators (e.g. killer whales) to track Blainville’s beaked whales acoustically.
Short-finned pilot whales and Blainville’s beaked whales also produce ultrasonic, click-based, pulsed calls or “rasps” during foraging dives. Rasps are comprised of a series of discrete, unmodulated, high repetition rate clicks. However, rasps are significantly shorter in duration than buzz clicks, which are echolocation sounds made during final prey capture. Rasp clicks are also produced at a slower rate than buzz clicks and lack the dramatic change in output level and rapid decrease in inter-click interval that occur during prey-capture attempts. For short-finned pilot whales, the occurrence of rasps increases at depth, which suggests that these signals may serve a communicative purpose[29]Pérez, J. M., Jensen, F. H., Rojano-Doñate, L., & Aguilar de Soto, N. (2017). Different modes of acoustic communication in deep-diving short-finned pilot whales ( Globicephala macrorhynchus ). Marine Mammal Science, 33(1), 59–79. https://doi.org/10.1111/mms.12344..
Additional Links on DOSITS
- Animals > Use of Sound > Marine Mammal Communication
- Animals > Use of Sound > Marine Mammal Navigation
- Animals > Use of Sound > Marine Mammal Foraging
- Animals > Use of Sound > Marine Mammal Reproduction
- Animals > Sound Reception > How do marine mammals hear?
- Animals > Sound Production > How do marine mammals produce sound?
- Technology Gallery > Acoustic Recording Tags
- Audio Gallery > Bottlenose dolphin
- Audio Gallery > Humpback whale
- Audio Gallery > Common dolphin
- Audio Gallery > Beluga whale
- Audio Gallery > Harbor porpoise
- Audio Gallery > Indo-Pacific humpback dolphin
- Audio Gallery > Killer whale
- Audio Gallery > Sperm whale
- Audio Gallery > Spinner dolphin
- Audio Gallery > North Atlantic right whale
- Audio Gallery > Bowhead whale
- Audio Gallery > Short-finned pilot whale
- Audio Gallery > Beaked whales
Additional Resources
- The Dolphin Communication Project: https://www.dolphincommunicationproject.org/
References
- Blomqvist, C., & Amundin, M. (2004). High-Frequency Burst-Pulse Sounds in Agonistic/Aggressive Interactions in Bottlenose Dolphins, Tursiops truncatus. In Echolocation in Bats and Dolphins (pp. 425–431). The University of Chicago Press.
- Caldwell, M. C., & Caldwell, D. K. (1971). Statistical evidence for individual signature whistles in the Pacific whitesided dolphin, Lagenorhynchus obliquidens. Cetology, 3, 1–9.
- Caldwell, M. C., Caldwell, D. K., & Miller, J. F. (1973). Statistical evidence for individual signature whistles in the spotted dolphin, Stenella plagiodon. Cetology, 16, 1–21.
- Fripp, D., Owen, C., Quintana-Rizzo, E., Shapiro, A., Buckstaff, K., Jankowski, K., Wells, R., & Tyack, P. (2005). Bottlenose dolphin (Tursiops truncatus) calves appear to model their signature whistles on the signature whistles of community members. Animal Cognition, 8(1), 17–26. https://doi.org/10.1007/s10071-004-0225-z.
- Herzing, D. L. (2000). Acoustics and Social Behavior of Wild Dolphins: Implications for a Sound Society. In W. W. L. Au, R. R. Fay, & A. N. Popper (Eds.), Hearing by Whales and Dolphins (Vol. 12, pp. 225–272). Springer New York. https://doi.org/10.1007/978-1-4612-1150-1_5.
- Herzing, D. L. (2014). Clicks, whistles and pulses: Passive and active signal use in dolphin communication. Acta Astronautica, 105(2), 534–537. https://doi.org/10.1016/j.actaastro.2014.07.003.
- Janik, V. M., & Sayigh, L. S. (2013). Communication in bottlenose dolphins: 50 years of signature whistle research. Journal of Comparative Physiology A, 199(6), 479–489. https://doi.org/10.1007/s00359-013-0817-7.
- Janik, V. M., Sayigh, L. S., & Wells, R. S. (2006). Signature whistle shape conveys identity information to bottlenose dolphins. Proceedings of the National Academy of Sciences, 103(21), 8293–8297. https://doi.org/10.1073/pnas.0509918103.
- Jones, B., Zapetis, M., Samuelson, M. M., & Ridgway, S. (2020). Sounds produced by bottlenose dolphins (Tursiops): A review of the defining characteristics and acoustic criteria of the dolphin vocal repertoire. Bioacoustics, 29(4), 399–440. https://doi.org/10.1080/09524622.2019.1613265.
- King, S. L., Sayigh, L. S., Wells, R. S., Fellner, W., & Janik, V. M. (2013). Vocal copying of individually distinctive signature whistles in bottlenose dolphins. Proceedings of the Royal Society B: Biological Sciences, 280(1757), 20130053–20130053. https://doi.org/10.1098/rspb.2013.0053.
- Lammers, M. O., Au, W. W. L., & Herzing, D. L. (2003). The broadband social acoustic signaling behavior of spinner and spotted dolphins. The Journal of the Acoustical Society of America, 114(3), 1629–1639. https://doi.org/10.1121/1.1596173.
- Mishima, Y., Morisaka, T., Itoh, M., Matsuo, I., Sakaguchi, A., & Miyamoto, Y. (2015). Individuality embedded in the isolation calls of captive beluga whales (Delphinapterus leucas). Zoological Letters, 1(1), 27. https://doi.org/10.1186/s40851-015-0028-x.
- Nielsen, M. L. K., Bejder, L., Videsen, S. K. A., Christiansen, F., & Madsen, P. T. (2019). Acoustic crypsis in southern right whale mother–calf pairs: Infrequent, low-output calls to avoid predation? The Journal of Experimental Biology, 222(13), jeb190728. https://doi.org/10.1242/jeb.190728.
- Sayigh, L. S. (2014). Cetacean Acoustic Communication. In G. Witzany (Ed.), Biocommunication of Animals (pp. 275–297). Springer Netherlands. https://doi.org/10.1007/978-94-007-7414-8_16.
- Tyack, P. L. (2000). Functional Aspects of Cetacean Communication. In J. Mann, R. C. Connor, P. L. Tyack, & H. Whitehead (Eds.), Cetacean Societies: Field Studies of Dolphins and Whales (pp. 270–307). University of Chicago Press.
- Tyack, P. L., & Miller, E. (2002). Vocal anatomy communication and echolocation. In A. R. Hoelzel (Ed.), Marine Mammal Biology an Evolutionary Approach (pp. 142–184). Blackwell Science.
- Videsen, S. K. A., Bejder, L., Johnson, M., & Madsen, P. T. (2017). High suckling rates and acoustic crypsis of humpback whale neonates maximise potential for mother–calf energy transfer. Functional Ecology, 31(8), 1561–1573. https://doi.org/10.1111/1365-2435.12871.
-
Walmsley, S. F., Rendell, L., Hussey, N. E., & Marcoux, M. (2020). Vocal sequences in narwhals ( Monodon monoceros ). The Journal of the Acoustical Society of America, 147(2), 1078–1091. https://doi.org/10.1121/10.0000671.
Cited References
| ⇡1 | Xitco, M. J., & Roitblat, H. L. (1996). Object recognition through eavesdropping: Passive echolocation in bottlenose dolphins. Animal Learning & Behavior, 24(4), 355–365. https://doi.org/10.3758/BF03199007 |
|---|---|
| ⇡2 | Gregg, J. D., Dudzinski, K. M., & Smith, H. V. (2007). Do dolphins eavesdrop on the echolocation signals of conspecifics? International Journal of Comparative Psychology, 20(1). https://escholarship.org/uc/item/20s5h7h9 |
| ⇡3 | Watkins, W. A., & Schevill, W. E. (1977). Sperm whale codas. The Journal of the Acoustical Society of America, 62(6), 1485. https://doi.org/10.1121/1.381678 |
| ⇡4 | Barbosa, M., Bittencourt, L., G. Andrade, L., L. Bisi, T., Lailson-Brito, J., & F. Azevedo, A. (2019). High-frequency social communication in Sotalia guianensis. The Journal of the Acoustical Society of America, 146(2), EL124–EL128. https://doi.org/10.1121/1.5120550. |
| ⇡5 | Oswald, J. N., Rankin, S., Barlow, J., & Lammers, M. O. (2007). A tool for real-time acoustic species identification of delphinid whistles. The Journal of the Acoustical Society of America, 122(1), 587–595. https://doi.org/10.1121/1.2743157. |
| ⇡6 | Sayigh, L. S., Tyack, P. L., Wells, R. S., Solow, A. R., Scott, M. D., & Irvine, A. B. (1999). Individual recognition in wild bottlenose dolphins: A field test using playback experiments. Animal Behaviour, 57(1), 41–50. https://doi.org/10.1006/anbe.1998.0961. |
| ⇡7 | Tyack, P. L., & Clark, C. W. (2000). Communication and Acoustic Behavior of Dolphins and Whales. In W. W. L. Au, R. R. Fay, & A. N. Popper (Eds.), Hearing by Whales and Dolphins (Vol. 12, pp. 156–224). Springer New York. https://doi.org/10.1007/978-1-4612-1150-1_4. |
| ⇡8 | King, S. L., Harley, H. E., & Janik, V. M. (2014). The role of signature whistle matching in bottlenose dolphins, Tursiops truncatus. Animal Behaviour, 96, 79–86. https://doi.org/10.1016/j.anbehav.2014.07.019. |
| ⇡9 | Kuczaj, S., Eskelinen, H., Jones, B., & Borger-Turner, J. (2015). Gotta Go, Mom’s Calling: Dolphin (Tursiops truncatus) Mothers Use Individually Distinctive Acoustic Signals To Call Their Calves. Animal Behavior and Cognition, 2(1), 88–95. https://doi.org/10.12966/abc.02.07.2015. |
| ⇡10 | King, S. L., Guarino, E., Keaton, L., Erb, L., & Jaakkola, K. (2016). Maternal signature whistle use aids mother-calf reunions in a bottlenose dolphin, Tursiops truncatus. Behavioural Processes, 126, 64–70. https://doi.org/10.1016/j.beproc.2016.03.005. |
| ⇡11 | Shapiro, A. D. (2006). Preliminary evidence for signature vocalizations among free-ranging narwhals (Monodon monoceros). The Journal of the Acoustical Society of America, 120(3), 1695–1705. https://doi.org/10.1121/1.2226586. |
| ⇡12 | Marcoux, M., Auger-Méthé, M., & Humphries, M. M. (2012). Variability and context specificity of narwhal (Monodon monoceros) whistles and pulsed calls. Marine Mammal Science, 28(4), 649–665. https://doi.org/10.1111/j.1748-7692.2011.00514.x. |
| ⇡13 | Morisaka, T., Yoshida, Y., Akune, Y., Mishima, H., & Nishimoto, S. (2013). Exchange of “signature” calls in captive belugas (Delphinapterus leucas). Journal of Ethology, 31(2), 141–149. https://doi.org/10.1007/s10164-013-0358-0. |
| ⇡14 | Vergara, V., & Mikus, M. (2019). Contact call diversity in natural beluga entrapments in an Arctic estuary: Preliminary evidence of vocal signatures in wild belugas. Marine Mammal Science, 35(2), 434–465. https://doi.org/10.1111/mms.12538 |
| ⇡15 | Vergara, V., & Mikus, M. (2019). Contact call diversity in natural beluga entrapments in an Arctic estuary: Preliminary evidence of vocal signatures in wild belugas. Marine Mammal Science, 35(2), 434–465. https://doi.org/10.1111/mms.12538 |
| ⇡16 | Cusano, D. A., Conger, L. A., Van Parijs, S. M., & Parks, S. E. (2019). Implementing conservation measures for the North Atlantic right whale: Considering the behavioral ontogeny of mother‐calf pairs. Animal Conservation, 22(3), 228–237. https://doi.org/10.1111/acv.12457. |
| ⇡17 | Díaz López, B., & Shirai, J. A. B. (2009). Mediterranean common bottlenose dolphin’s repertoire and communication use. In A. G. Pearce & L. M. Correa (Eds.), Dolphins: Anatomy, Behavior, and Threats. (pp. 129–148). Nova Science Publishers, Inc. |
| ⇡18 | Simard, P., Mann, D. A., & Gowans, S. (2008). Burst-Pulse Sounds Recorded from White-Beaked Dolphins (Lagenorhynchus albirostris). Aquatic Mammals, 34(4), 464–470. https://doi.org/10.1578/AM.34.4.2008.464. |
| ⇡19 | Rankin, S., Oswald, J. N., Simonis, A. E., & Barlow, J. (2015). Vocalizations of the rough-toothed dolphin, Steno bredanensis , in the Pacific Ocean. Marine Mammal Science, 31(4), 1538–1548. https://doi.org/10.1111/mms.12226. |
| ⇡20 | Rendell, L. E., & Whitehead, H. (2003). Vocal clans in sperm whales (Physeter macrocephalus). Proceedings of the Royal Society B: Biological Sciences, 270(1512), 225–231. https://doi.org/10.1098/rspb.2002.2239. |
| ⇡21 | Oliveira, C., Wahlberg, M., Silva, M. A., Johnson, M., Antunes, R., Wisniewska, D. M., Fais, A., Gonçalves, J., & Madsen, P. T. (2016). Sperm whale codas may encode individuality as well as clan identity. The Journal of the Acoustical Society of America, 139(5), 2860–2869. https://doi.org/10.1121/1.4949478 |
| ⇡22 | Gero, S., Whitehead, H., & Rendell, L. (2016). Individual, unit and vocal clan level identity cues in sperm whale codas. Royal Society Open Science, 3(1), 150372. https://doi.org/10.1098/rsos.150372. |
| ⇡23, ⇡25 | Sørensen, P. M., Wisniewska, D. M., Jensen, F. H., Johnson, M., Teilmann, J., & Madsen, P. T. (2018). Click communication in wild harbour porpoises (Phocoena phocoena). Scientific Reports, 8(1), 9702. https://doi.org/10.1038/s41598-018-28022-8. |
| ⇡24 | Clausen, K. T., Wahlberg, M., Beedholm, K., Deruiter, S., & Madsen, P. T. (2011). Click communication in harbour porpoises Phocoena phocoena. Bioacoustics, 20(1), 1–28. https://doi.org/10.1080/09524622.2011.9753630. |
| ⇡26, ⇡27 | Jensen, F. H., Perez, J. M., Johnson, M., Soto, N. A., & Madsen, P. T. (2011). Calling under pressure: Short-finned pilot whales make social calls during deep foraging dives. Proceedings of the Royal Society B: Biological Sciences, 278(1721), 3017–3025. https://doi.org/10.1098/rspb.2010.2604. |
| ⇡28 | Aguilar de Soto, N., Madsen, P. T., Tyack, P., Arranz, P., Marrero, J., Fais, A., Revelli, E., & Johnson, M. (2012). No shallow talk: Cryptic strategy in the vocal communication of Blainville’s beaked whales. Marine Mammal Science, 28(2), E75–E92. https://doi.org/10.1111/j.1748-7692.2011.00495.x. |
| ⇡29 | Pérez, J. M., Jensen, F. H., Rojano-Doñate, L., & Aguilar de Soto, N. (2017). Different modes of acoustic communication in deep-diving short-finned pilot whales ( Globicephala macrorhynchus ). Marine Mammal Science, 33(1), 59–79. https://doi.org/10.1111/mms.12344. |